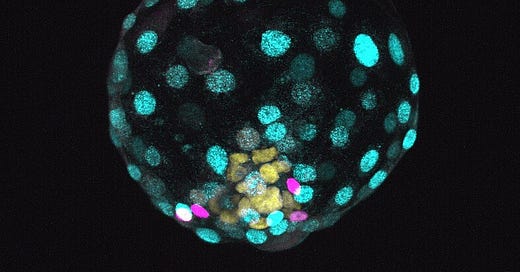
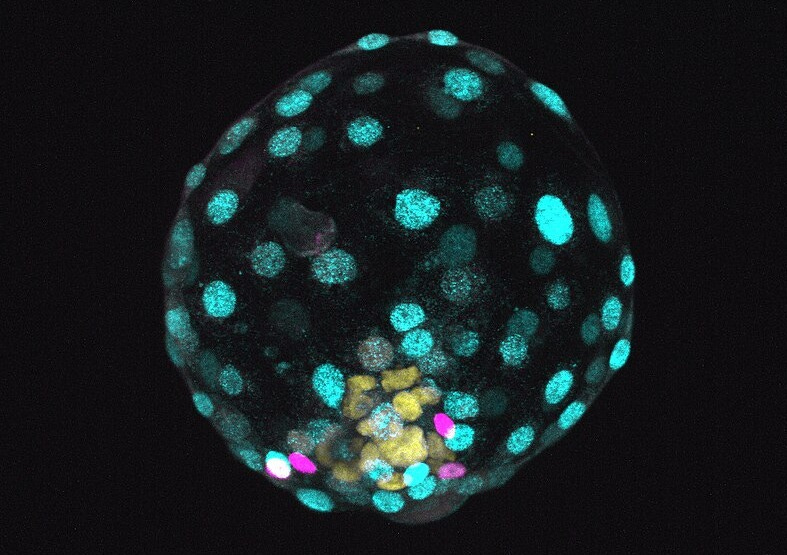
Spurred by a series of remarkable discoveries pioneered by two laboratories in Cambridge, UK, and Israel, recent reports about embryo models, occasionally sensationalized as 'synthetic embryos,' have stirred significant discussion within both the scientific community and the public sphere. As anticipated, these discussions have oscillated between undertones of moral panic and moments of rigorous ethical deliberation. Essentially, embryo models are three-dimensional assemblies derived from pluripotent stem cells, which self-organize to mirror structural and functional features observed in embryos. These models can mimic embryo development during the pre- and early post-implantation stages of human life, and their current state of development has culminated in entities that resemble the structural organization and morphology of a human embryo at day 14. Notably, these models can be studied outside the uterus, obviating the necessity for implantation, and can be formed in vitro directly from stem cells, effectively bypassing the need for egg and sperm. Below is a video displaying a mouse embryo model at day 8 of development, featuring a beating heart (from Amadei et al.).
Embryo models hold immense promise for propelling biomedical research, for example by serving as tools in disease modeling and drug discovery. They present a promising alternative to conventional animal testing approaches, notably in areas such as toxicology and teratogenicity screening. Perhaps most intriguingly, these models grant researchers unprecedented experimental access to embryonic developmental stages that were previously inaccessible. Surplus embryos from in vitro fertilization (IVF) procedures are typically cryopreserved at the early blastocyst stage, around the fifth or sixth day, and the generation of embryos specifically for research is only allowed in a few jurisdictions. Hence, studying early embryonic development presents significant challenges, leaving our understanding of the subject in a rudimentary stage. Furthermore, most major jurisdictions prohibit culturing embryos in the laboratory beyond 14 days, which leaves critical stages of embryonic development, such as gastrulation, more or less shrouded in mystery. Gaining insight into these previously opaque stages of embryo development not only fuels theoretical curiosity but also carries significant implications for reproductive health and fertility research. For example, such understanding is crucial for comprehending the susceptibility to pregnancy loss or the developmental origins of health and disease.
The prevailing response to the first reports of embryo models was primarily one of relief, as the exploration of these models offers a potential avenue to navigate ethical complexities and circumvent existing restrictions tied to human embryo research. Indeed, in light of current technological capabilities, it seems more than reasonable to assess the use of embryo models through a lens of utility and pragmatism. These models act as proxies for investigating very specific questions in biology, each varying in completeness and recapitulating different developmental stages. The International Society for Stem Cell Research (ISSCR) advocates for different levels of ethical scrutiny and oversight, distinguishing between 'non-integrated' models, which support the formation of discrete anatomical structures but have limited biological viability, and 'integrated' models, incorporating both embryonic and extraembryonic tissues, capable of sustained in vitro culture and more accurate development.
However, while integrated embryo models currently only replicate certain embryonal structures, they are on a trajectory to become ever more akin to natural embryos. At this juncture, things start to get hairy. The pragmatic approach outlined above operates on the assumption that such models currently lack the moral sensitivities attributed to embryos. As dissimilarities dwindle, the arguments typically propose that closer ethical scrutiny is necessary, and at certain 'tipping points,' these models should be accorded the full status of embryos. Yet, the rationale behind both the current differentiation and the definition of potential tipping points remains somewhat obscure, and upon closer examination, it appears to recycle familiar yet not particularly enlightening arguments concerning the moral status of embryos, notably the 'potentiality argument.'
First of all, the concept of moral status itself remains rather elusive, and the very foundation of what confers it is hotly contested. Some argue that mere membership in the species Homo sapiens suffices, while others hold that specific capacities, such as sentience, are pivotal. Furthermore, identifying the precise features that confer status to other embryo-like entities is not only a daunting task but also presents both theoretical and practical conundrums. Villalba et al. highlight a theoretical paradox in embryo modeling: such models may either become too akin to natural embryos, rendering their existence redundant, or they may remain dissimilar, inadequate in fulfilling the expectations placed upon them. This, in turn, prompts questions about their utilization as scientific tools, given the uncertainty regarding their moral equivalence to human embryos on the one hand, and their scientific utility on the other. Rivron et al. highlight a very practical challenge: the most rigorous method for assessing the innate potential of embryo models, namely evaluating their viability through transfer to a uterus, is rightfully prohibited due to profound ethical concerns. It is unlikely that a method both ethically justified and safe will emerge in the near future to make this option viable.
This has prompted many to resort to approximations for defining status based on the developmental potential of cells to progress into a fetus. Villalba et al. propose a combinatorial approach wherein the similarity to embryos should be assessed based on specific features or combinations thereof. They suggest that the accurate replication of cardiac and neural structures, akin to those found in fetuses beyond abortion redlines, are hot contenders for conferring embryo-like moral status. A similar approach is proposed by Rivron et al., suggesting a dual-pronged 'Turing test' to determine the equivalence of models to embryos. They propose that models should be considered equivalent to embryos when: (1) they demonstrate the potential to develop efficiently and faithfully in vitro, resembling normal development up to a given stage of development based on local ethical and regulatory guidelines, and (2) equivalent animal embryo models show the potential to produce living and fertile animals across multiple species, including those closest to humans (e.g., pigs, monkeys).
These approaches seem reasonable for governing the use of embryo models in practice. In today's context, where frozen embryos can be recognized as children in certain Western jurisdictions, it is prudent to avoid invoking parental sentiments towards these entities by drawing parallels to embryos. However, it's essential to recognize the reality of embryo models: rather than representing a genuine moral advantage over the use of embryos, they primarily serve as a workaround within flawed regulatory systems, as aptly articulated by Gyngell et al. The attempt to portray the use of embryo models as morally superior to embryos is, in fact, a moral obfuscation.
Essentially, a commonly cited attribute believed to be relevant to moral status is the potential of an entity to develop into a human being. This capacity to culminate in a live birth is conceivably present in embryo models. However, as Gyngell et al. remind us, the normative assertions posited by the potentiality argument have faced critique in scholarly discourse. One aspect of contention lies in the ubiquity of potentiality, given that any human nucleated cell can be reprogrammed into a pluripotent cell, and most of these cells are deemed morally insignificant. Another issue relates to logical reasoning:
“Just as acorns are distinct from oak trees and eggs are not the same as chickens or omelettes, we should not confuse potentiality with actuality. The mere possibility or probability of a transformation does not mean we should treat something as if it has already been transformed. Similarly, while it is true that we all have the potential to die, given that immortality is unattainable, it does not mean we should be treated as if we are already deceased.”
Efforts to counter this argument have indeed emerged, often by distinguishing between 'active' versus 'passive' or 'intrinsic' versus 'extrinsic' potential. The rationale behind this is to assert that embryos possess inherent factors that confer upon them the capacity for developmental progression to birth, thus endowing them with moral status, unlike cells requiring external manipulation to fulfill such potential. However, this line of reasoning fails to persuade convincingly; ubiquity considerations are equally applicable here. All biological entities inevitably interact with their environment, and their future development hinges upon these interactions. For instance, surplus IVF embryos necessitate a very specific sequence of actions and interventions to have any chance of forming a viable pregnancy. Similar reasoning applies to other iterations of this argument, such as substituting 'potential' with the 'likelihood' of becoming a person. While the latter is minimal for a somatic cell, it remains equally marginal for a surplus IVF embryo designated for research purposes.
Gyngell et al.'s sharp analysis really hits the bullseye: it's tough to pinpoint any intrinsic or biological disparity between embryos and embryo models that justifies elevating embryos to a higher moral status. If you want to clinch an argumentative point, mere potentiality doesn't make the cut. While on the surface, embryo models may boast practical advantages over embryos, such as the potential to generate a huge number of genetically identical models, these advantages would likely dissipate if we could create, clone, and genetically manipulate embryos for research purposes. In essence, embryo models serve as practical expedients within flawed regulatory frameworks, rather than heralding a moral leap beyond the use of embryos.
Public outreach raises a specific concern regarding the delicate balance of engaging laypeople without unintentionally patronizing them, a consideration that ought to evoke a sense of unease among scientists and ethicists. Occasionally, by unnecessarily complicating the issue and presenting embryo models as fundamentally distinct from embryos, ethicists and scientists may inadvertently overlook their profound similarities, thus obscuring some straightforward ethical considerations from plain view. Engaging in such candid conversations might indeed evoke public unease about embryo models, a reaction practitioners may seek to avoid. However, embracing this more forthright approach is ultimately preferable, as it offers the opportunity to address concerns regarding the use of embryos for purposes aligned with those of embryo models.
While advocating for responsible research practices, it's crucial to emphasize that research involving embryos or advanced embryo models shouldn't resemble the untamed frontier with no regulations whatsoever, just because we can’t agree on status questions. On the contrary, considerations such as their capacity for sentience or ability to experience pain are relevant—it's just that these ethical considerations apply equally to both embryos and embryo models.